New and Improved Food Labels Are Here!
The Food and Drug Administration of the Department of Health and Human Services and the Food Safety and Inspection Service of the U.S. Department of Agriculture have updated the Nutrition Facts section of the food label as of January 2006. Under the new guidelines, manufacturers must list trans fat in addition to saturated fat and cholesterol. Trans fat- the type of fat listed in ingredients as ‘hydrogenated’ or ‘partially hydrogenated’ fat has been linked with the development of coronary artery disease because it raises blood cholesterol levels.
Nutrition Information Panel
Under the label’s “Nutrition Facts” panel, manufacturers are required to provide information about certain nutrients. The mandatory (Bolded) and voluntary components and the order in which they have to appear are:
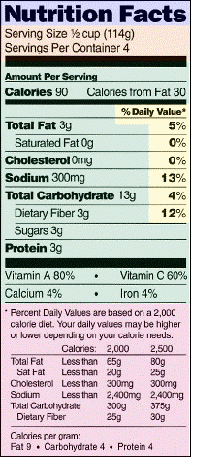 |
|
Additional information about a food’s nutritional content can be listed on the Nutrition Facts label with specific guidelines. For example, voluntary information about types of fiber, polyunsaturated fat or potassium can be included. Foods fortified with other nutrients must list these nutrients on the label. If a claim is made about any of the optional nutrients, (such as folate and birth defects), or if a food is fortified with additional nutrients, information must be provided on the label.
The FDA is also amending the voluntary nutrition labeling regulations by updating the names and the nutrition labeling values for the 20 most frequently consumed raw fruits, vegetables, and fish in the United States and clarifying guidelines for the voluntary nutrition labeling of these foods. Availability of the updated nutrition labeling values in retail stores and on individually packaged raw fruits, vegetables, and fish will enable consumers to make better purchasing decisions to reflect their dietary needs.
Daily Reference Values–DRVs
DRVs have been established for macronutrients that are sources of calories and include fat, saturated fat, total carbohydrate (including fiber), and protein. DRVs have also been developed for cholesterol, sodium and potassium, which do not contribute calories.
DRVs for the calorie-containing nutrients are based on the number of calories consumed per day. A daily intake of 2,000 calories has been established as the reference. This level was chosen, because it approximates the caloric requirements for postmenopausal women. This population is most at risk for excessive intake of calories and fat.
DRVs for the calorie-containing nutrients are calculated as follows:
- Fat based on 30 percent of calories
- Saturated fat based on 10 percent of calories
- Carbohydrate based on 60 percent of calories
- Protein based on 10 percent of calories. (The DRV for protein applies only to adults and children over 4).
- Fiber based on 11.5 g of fiber per 1,000 calories.
Nutrition Panel Format
All nutrients must be declared as percentages of the Daily Values. These are label reference values. The amount of macronutrients (fat, carbohydrate, protein, cholesterol, and sodium) are listed to the immediate right of these nutrients in grams or milligrams. For the first time, a column headed “% Daily Value” appears on the far right side.
Quantifying nutrients as a percentage of the Daily Values is intended to prevent confusion that may arise with numeric values. For example, a food with 140 milligrams (mg) of sodium could be mistaken for a high-sodium food because 140 is a relatively large number. In reality, that amount represents less than 6 percent of the Daily Value of 2,400 mg for sodium.
On the other hand, a food with 5 g of saturated fat could be seen as being low in that nutrient. In actuality, that food would provide one-fourth the total Daily Value because 20 g is the Daily Value for saturated fat. The % Daily Value listing carries a footnote stating that the percentages are based on a 2,000-calorie diet.
Serving Sizes
The serving size is used for reporting a food’s nutrient content. In the past, the serving size was up to the discretion of the food manufacturer. Now serving sizes are more uniform and reflect the amounts people actually eat. Future labeling laws may make nutrition information even easier for consumers to understand. For example, a 20 oz. bottle of Coke is currently considered to contain 2 ½ servings. However, since most people drink the entire bottle, one serving would be 250 calories (versus 100 calories for one serving). According to a new FDA task force designed to combat obesity, food manufacturers will be asked to label as a single serving those food packages where the entire content of the package can reasonably be consumed on a single eating occasion. If companies do not comply, they could face fines and other penalties.
FDA allows the following common household measures: the cup, tablespoon, teaspoon, piece, slice, fraction (such as “1/4 pizza”), and common household containers used to package food products (such as a jar or tray). Ounces may be used only if a common household unit is not applicable and an appropriate visual unit is given–for example, 1 oz (28g/about 1/2 pickle).
Grams (g) and milliliters (mL) are the metric units that are used in serving size statements.
Claims for Nutrient Content
The regulations also indicate what terms can be used to describe the level of a nutrient in a food and how they can be used. These are the core terms:
- Free. This term means that a food contains no amount of, or only trivial or “physiologically inconsequential” amounts of, one or more of these components: fat, saturated fat, trans fat, cholesterol, sodium, sugars, and calories. For example, “calorie-free” means fewer than 5 calories per serving, and “sugar-free” and “fat-free” both mean less than 0.5 g per serving. Other terms for “free” include “without,” “no” and “zero.” A synonym for fat-free milk is “skim”.
-
Low. This term can be used on products that can be eaten frequently without exceeding dietary guidelines for one or more of these components: fat, saturated fat, cholesterol, sodium, and calories. Thus, descriptors are defined as follows:
- low-fat: 3 g or less per serving
- low-saturated fat: 1 g or less per serving
- low trans fat: < .5 g or less per serving
- low-sodium: 140 mg or less per serving
- very low sodium: 35 mg or less per serving
- low-cholesterol: 20 mg or less and 2 g or less of saturated fat per serving
- low-calorie: 40 calories or less per serving.
Other terms for low include “little,” “few,” “low source of,” and “contains a small amount of.”
-
Lean and extra lean. These terms can be used to describe the fat content of meat, poultry, seafood, and game meats.
- lean: less than 10 g fat, 4.5 g or less saturated fat, and less than 95 mg cholesterol per serving and per 100 g.
- extra lean: less than 5 g fat, less than 2 g saturated fat, and less than 95 mg cholesterol per serving and per 100 g.
- High. This term can be used if the food contains 20 percent or more of the Daily Value for a particular nutrient in a serving.
- Good source. This term means that one serving of a food contains 10 to 19 percent of the Daily Value for a particular nutrient.
- Reduced. This term means that a nutritionally altered product contains at least 25 percent less of a nutrient or of calories than the regular, or reference, product. However, a reduced claim can’t be made on a product if its reference food already meets the requirement for a “low” claim.
- Less. This term means that a food, whether altered or not, contains 25 percent less of a nutrient or of calories than the reference food. For example, pretzels that have 25 percent less fat than potato chips could carry a “less” claim. “Fewer” is an acceptable synonym.
-
Light. This descriptor can mean two things:
- First, that a nutritionally altered product contains one-third fewer calories or half the fat of the reference food. If the food derives 50 percent or more of its calories from fat, the reduction must be 50 percent of the fat.
- Second, that the sodium content of a low-calorie, low-fat food has been reduced by 50 percent. In addition, “light in sodium” may be used on food in which the sodium content has been reduced by at least 50 percent.
The term “light” can also be used to describe properties such as texture and color, as long as the label explains the intent–for example, “light brown sugar” and “light and fluffy.”
- More. This term means that a serving of food, whether altered or not, contains a nutrient that is at least 10 percent of the Daily Value more than the reference food. The 10 percent of Daily Value also applies to “fortified,” “enriched” and “added” “extra and plus” claims. In those cases, the food must be altered.
- Healthy. A “healthy” food must be low in fat and saturated fat and contain limited amounts of cholesterol and sodium. If it’s a single-item food, it must provide at least 10 percent of one or more of vitamins A or C, iron, calcium, protein, or fiber. Certain raw, canned and frozen fruits and vegetables and certain cereal-grain products are exempt from this “10-percent” rule. These foods can be labeled “healthy,” if they do not contain ingredients that change the nutritional profile, and, in the case of enriched grain products, conform to standards of identity, which call for certain required ingredients. Frozen entrees and multi-course frozen dinners or other meal-type entrees must provide 10 percent of two or three of these vitamins or minerals or of protein or fiber, plus meet the other criteria. The sodium content cannot exceed 360 mg per serving for individual foods and 480 mg per serving for meal-type products.
- Because of the surge in interest in carbohydrates, the FDA will start defining what foods can be labeled “low,” “reduced” or “free” of carbs.
Baby Foods
FDA is not allowing broad use of nutrient claims on infant and toddler foods. However, the agency may propose claims specifically for these foods at a later date. The terms “unsweetened” and “unsalted” are allowed on these foods, because they relate to taste and not nutrient content
Health Claims
Claims for 10 relationships between a nutrient or a food and the risk of a disease or health-related condition are currently allowed. They can be made in several ways: through third-party references (such as the National Cancer Institute), statements, symbols (such as a heart), and vignettes or descriptions. The claim must meet the requirements for authorized health claims–for example, they cannot state the degree of risk reduction and can only use “may” or “might” in discussing the nutrient or food-disease relationship. In addition, they must state that other factors play a role in that disease.
The claims also must be written so that consumers can understand the relationship between the nutrient and the disease and the importance of that nutrient in the daily diet.
An example of an appropriate claim is: “While many factors affect heart disease, diets low in saturated fat and cholesterol may reduce the risk of this disease.”
The allowed nutrient-disease relationship claims and rules for their use are:
- Calcium and osteoporosis: To carry this claim, a food must contain 20 percent or more of the Daily Value for calcium (200 mg) per serving, have a calcium content that equals or exceeds the food’s content of phosphorus, and contain a form of calcium that can be readily absorbed and used by the body. The claim must name the population most in need of adequate calcium intakes (that is, teens and young adult white and Asian women) and state the need for exercise and a healthy diet. A product that contains 40 percent or more of the Daily Value for calcium must state on the label that a total dietary intake greater than 200 percent of the Daily Value for calcium (that is, 2,000 mg or more) has no further known benefit.
- Fat and cancer: To carry this claim, a food must meet the nutrient content claim requirements for “low-fat” or, if fish and game meats, for “extra lean.”
- Saturated fat and cholesterol and coronary heart disease (CHD): This claim may be used if the food meets the definitions for the nutrient content claim “low saturated fat,” “low-cholesterol,” and “low-fat,” or, if fish and game meats, for “extra lean.” It may discuss the link between reduced risk of CHD and lower saturated fat and cholesterol intakes to lower blood cholesterol levels.
- Fiber-containing grain products, fruits and vegetables and cancer: To carry this claim, a food must be or must contain a grain product, fruit or vegetable and meet the nutrient content claim requirements for “low-fat,” and, without fortification, be a “good source” of dietary fiber.
- Fruits, vegetables and grain products that contain fiber and risk of CHD: To carry this claim, a food must be or must contain fruits, vegetables and grain products. It also must meet the nutrient content claim requirements for “low saturated fat,” “low-cholesterol,” and “low-fat” and contain, without enrichment, at least 0.6 g soluble fiber per serving.
- Sodium and hypertension (high blood pressure): To carry this claim, a food must meet the nutrient content claim requirements for “low-sodium.”
- Fruits and vegetables and cancer: This claim may be made for fruits and vegetables that meet the nutrient content claim requirements for “low-fat” and that, without fortification, for “good source” of at least one of the following: dietary fiber or vitamins A or C. This claim relates diets low in fat and rich in fruits and vegetables (and thus vitamins A and C and dietary fiber) to lowered cancer risk. FDA authorized this claim in place of an antioxidant vitamin and cancer claim.
- Folic acid and neural tube defects: This claim is allowed on dietary supplements that contain sufficient folate and on conventional foods that are naturally good sources of folate, as long as they do not provide more than 100 percent of the Daily Value for vitamin A as retinol or preformed vitamin A or vitamin D. A sample claim is “healthful diets with adequate folate may reduce a woman’s risk of having a child with a brain or spinal cord defect.”
- Dietary sugar alcohols and dental caries (cavities): This claim applies to food products, such as candy or gum, containing the sugar alcohols xylitol, sorbitol, mannitol, maltitol, isomalt, lactitol, hydrogenated starch hydrolysates, hydrogenated glucose syrups, or a combination of any of these. If the food also contains a fermentable carbohydrate, such as sugar, the food cannot lower the pH of plaque in the mouth below 5.7. Besides the food ingredient’s relationship to dental caries, the claim also must state that frequent between-meal consumption of foods high in sugars and starches promotes tooth decay. A shortened claim is allowed on food packages with less than 15 square inches of labeling surface area.
- Soluble fiber from certain foods, such as whole oats and psyllium seed husk, and heart disease: This claim must state that the fiber also needs to be part of a diet low in saturated fat and cholesterol, and the food must provide sufficient soluble fiber. The amount of soluble fiber in a serving of the food must be listed on the Nutrition Facts panel.
- ” The FDA also planned to consider allowing companies to make health claims for some foods that meet the FDA’s definition of “reduced” or “low” in calories, such as: “Diets low in calories may reduce the risk of obesity, which is associated with type 2 diabetes, heart disease and cancers.”
Restaurant Foods
Nutrition information is now required for some restaurant foods. FDA requires nutrition information for foods in which health or nutrient-content claims are made on restaurant menus, signs or placards. Restaurants have to provide a “reasonable basis” for making claims, although they are given some flexibility in this. For example, they could rely on recipes endorsed by medical or dietary groups. The FDA is also considering requiring restaurants; especially chain restaurants with standardized portions and recipes, to more clearly label how many calories and fat are in each serving. This is one of the many efforts to help combat the growing problem of obesity in America.
Much of this information was taken from the web site www.fda.gov
For More Information
FDA
General Inquiries: Call toll-free 1-888-INFO-FDA (1-888-463-6332).
Food Safety Hotline: 1-800-332-4010
FDA’s food label information:http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/default.htm
USDA
Food Safety Education and Communication Office
1400 Independence Ave., S.W., Room 1180
Washington, DC 2025
Meat and Poultry Hotline: 1-800-535-4555.
For more information:
Go to the Diet and Nutrition health topic.